Macrocyclization-induce emission enhancement characteristics of
benzothiadiazole-based macrocycles: A theoretical study
Abstract: We present a comprehensive investigationinto the molecular structures in the ground state and the first singlet excitedstate, intramolecular and intermolecular interactions, as well as the emissionproperties of monomers, dimers and macrocycles using density functional theoryand time dependent density functional theory. Our study aims to unveil themacrocyclization induce emission enhancement principle forbenzothiadiazole based macrocycles. Compared with the typical ππ stacking ofmonomers, macrocycles exhibit the tighter stacking arrangement due to thepresence of strong intramolecular and intermolecular hydrogen bond interactionsand the rigid triangular geometry of macrocycle, which is conducive to theenhancement of emissions efficiency. The calculated oscillator strength ofmacrocycles in the first excited state is much higher than that of dimers composedof two monomers. Meanwhile, the large transition electric dipole moment isbeneficial for enhancing the emission efficiency. Additionally, our studyreveals an emission wavelength red shift for macrocycles after “CH”/N substitution in the acceptor moiety, and the transition modefrom LUMO to HOMO can be mainly attributed to intramolecular charge transitioncharacters from acceptor to donor fragments. Our theoretical study mightprovide valuable insights for the design of innovative luminescent macrocycleswith high emission efficiency.
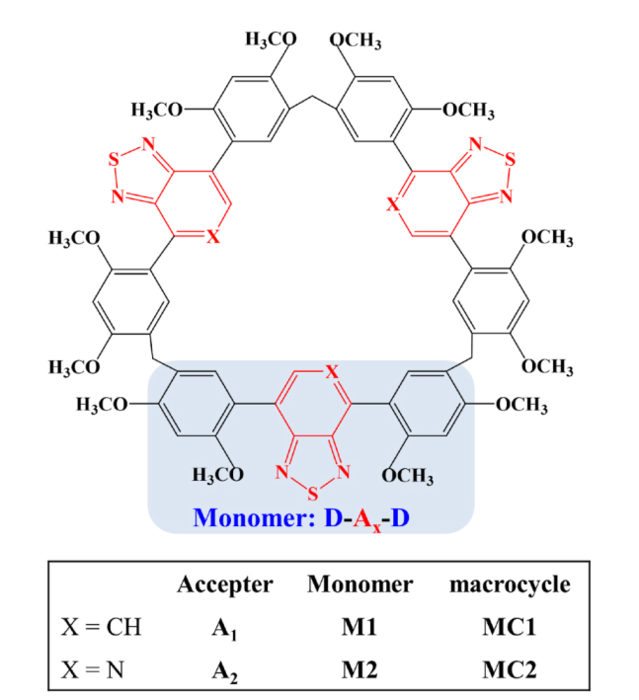
Jing Lu, Yi Sun, Yayun Hu, Chunliu He,kangyu Jia, Dong Wang,Macrocyclizationinduce emission enhancement characteristics of benzothiadiazole based macrocycles: A theoretical study,Journal of Luminescence ,Volume 267 ,2024 ,120374 ,ISSN0022.2313, https: //doi.org/10.1016/j.jlumin.2023.120374.